How to Avoid Over-the-Counter Hearing Aid Scams in 2025
Key Takeaways
- OTC hearing aid scams target unsuspecting consumers with false claims, misleading labels, and empty promises of money-back guarantees and customer support that companies don’t uphold.
- Because OTC hearing aids can be purchased without help from a hearing professional, it’s important to know how to spot the warning signs of a hearing aid scam.
- You can avoid becoming the victim of a scam by using the tips below to identify trusted companies that sell FDA-registered or FDA-cleared hearing aids.
If you’ve shopped for hearing aids recently, you may have been surprised by the sheer number of options. As hearing aid manufacturers work to align with the Food and Drug Administration’s (FDA) over-the-counter (OTC) hearing aid regulations and new companies enter the market, customers have more choices than ever before.
You can now buy OTC hearing aids for mild to moderate hearing loss online and in stores without a hearing exam or prescription. This means it’s critical for consumers to know how to separate reputable companies from fakes. Read on for ways to identify FDA-registered and FDA-cleared hearing aids, details on recent hearing aid regulation changes, and tips for avoiding scams from our team.
Top 7 OTC hearing aid scams to watch out for
With so many hearing aid brands and models to choose from, it can be tough to know which ones are reputable and trustworthy, let alone how to find the model that will best suit your needs.
By reading our tips on the seven most common hearing aid scams, you’ll know what to look for as you shop for hearing aids.
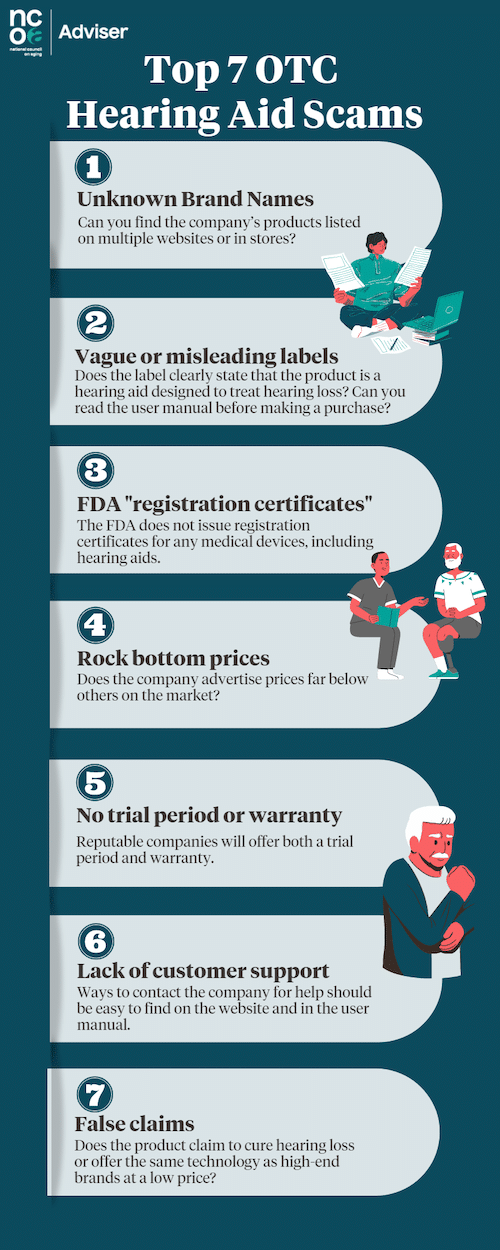
1. Unknown brand names
Be cautious if a company’s name is listed only on a single website, with no reviews or descriptions elsewhere. Most reputable OTC hearing aid companies will be reviewed and advertised on multiple third-party hearing and retail websites.
2. Vague or misleading labels
Be sure a device is clearly marked as a hearing aid. Some companies use vague or misleading labels like “hearing enhancement” to avoid declaring their products are not, in fact, hearing aids.
John Luna is the CEO of OTC hearing aid manufacturer Nuheara. He also acts as chair of the working committee on OTC hearing aids for the Consumer Technology Association and serves as chair of the Consumer Healthcare Products Association medical device committee.
Luna said the FDA’s final rule on OTC hearing aids is focused on keeping consumers safe when purchasing devices directly from manufacturers.1 One critical part of this goal is requiring transparency on websites and packaging.
“It’s all to protect the consumer, to make sure safety and efficacy are in place, and that the buyer has the ability to review the use and functionality of the product via the user manual and instructions for use,” said Luna.
Companies that don’t have “OTC hearing aids” printed on the label, packaging, or in the product description are not adhering to FDA OTC regulations for clear labeling. These products may actually be personal sound amplification products (PSAPs) rather than hearing aids.
Both PSAPs and hearing aids have their uses, but it’s important to know what you’re buying.
If you see language pointing to a product’s ability to treat hearing loss, help you hear in situations that people with hearing loss commonly have difficulty with, or be tailored to your hearing loss, that product must meet the FDA’s guidelines for hearing aids.
As Luna stated above, you should also be able to access the user manual online or in stores before making a purchase. Reading the instructions before buying a hearing aid will give you the opportunity to learn how to use it and determine whether you will need other devices to use it, such as a smartphone or hearing aid accessories, like an external microphone.
3. Fake FDA “registration certificates”
Any company that displays a certificate from the FDA is being misleading. The FDA does not issue registration certificates to medical device companies.2
4. Rock-bottom prices
Although the cost of hearing aids is dropping, any brand that sells products at prices far below the competition may be selling PSAPs rather than hearing aids. The allure of rock-bottom prices advertised on social media, especially Facebook, can be a trap.
Keep in mind that even among FDA-registered hearing aids, you get what you pay for. The most budget-friendly hearing aids we’ve found, including Audien and Go Hearing, are very basic compared to some of the higher-priced brands.
Many legitimate OTC hearing aids are much more affordable than prescription devices, and that doesn’t mean they’re necessarily bad. A low price by itself shouldn’t completely turn you off from a company, but it is one of the many potential warning factors.
While it’s important to consider your budget, you also want to make sure you’re happy with your purchase. Research has found one of the top reasons hearing aid users stop using their hearing aids is due to pain or a poor fit.3
Hearing aids can be very much like eyeglasses—a little discomfort in the morning can worsen as the day progresses, causing you to stop wearing them. If you can afford to, you may want to pay more for a better pair of hearing aids you’ll get the most use out of.
Hearing aid brands, especially lesser-known ones, often leverage social media platforms like Facebook to advertise their products directly to consumers. While convenient, these ads often prioritize eye-catching, low introductory prices over crucial information about the product’s features and functionalities. Be cautious of these “too-good-to-true” offers.
Nebroo Hearing Aids, a new company aggressively advertised on Facebook, offers budget-friendly options starting at $180 USD. However, their limited product information, questionable business practices, and aggressive marketing tactics raise concerns. For instance, they’re very vague about features and lack specifics on what their hearing aids include. Their privacy policy also allows unsolicited marketing and has concerning clauses restricting user communication.
5. No trial period or warranty
Most states require hearing aid manufacturers to provide a trial period of at least 30 days.4 During this time, you can return the hearing aids for a refund if they aren’t meeting your needs. All of the brands we’ve researched offer at least 30 days to try out new devices, and some (like Jabra Enhance) give you up to 100 days.
Be wary of any company that doesn’t provide a trial period. New hearing aid wearers need this time to adjust to their new devices, learn how to use the features, and get professional support if needed.
6. Lack of customer support
You should be able to find contact information on the website under “Customer Support” or “Contact Us.” It’s often listed at the top or bottom of the page. If you can’t find it easily, try searching the site for “contact” or “help.” A reputable company will make it straightforward to reach out.
If the company doesn’t offer an easy way to contact someone and ask questions, it’s best to move on. A reputable company will prioritize accessible and responsive customer support.
A quality company will also respond in a reasonable timeframe. If a company has contact information but takes several business days to reply, it could be a red flag. Scam companies may have customer service email addresses that are not monitored.
7. Unsubstantiated claims
Watch out for language stating a product can “cure” hearing loss, will give you “immediate relief from symptoms,” or has the same technology as high-priced hearing aids at a fraction of the price.
Hearing aids will not completely restore your hearing to its previous level, and there is no cure for hearing loss.5 They can greatly improve your hearing ability, though, if worn consistently. But, any claims that hearing aids are a cure-all for hearing loss are false.
Keep in mind advanced technology will cost more. For example, hearing aids offering Bluetooth streaming and hands-free calls start at $800–$1,000 per pair, based on our team’s research. And high-tech hearing aids that automatically adjust to your sound environment also sell for that much or more. Be skeptical of any claims promising leading-edge technology and features at a low price.

If you’ve been targeted by a hearing aid scam, you can report it to the FDA.6 By doing so, you’ll be protecting yourself and others from being taken advantage of in the future.
How to avoid OTC hearing aid scams
In addition to checking for the above red flags that can alert you to a scam, follow these tips to make sure you don’t get taken advantage of by unscrupulous companies.
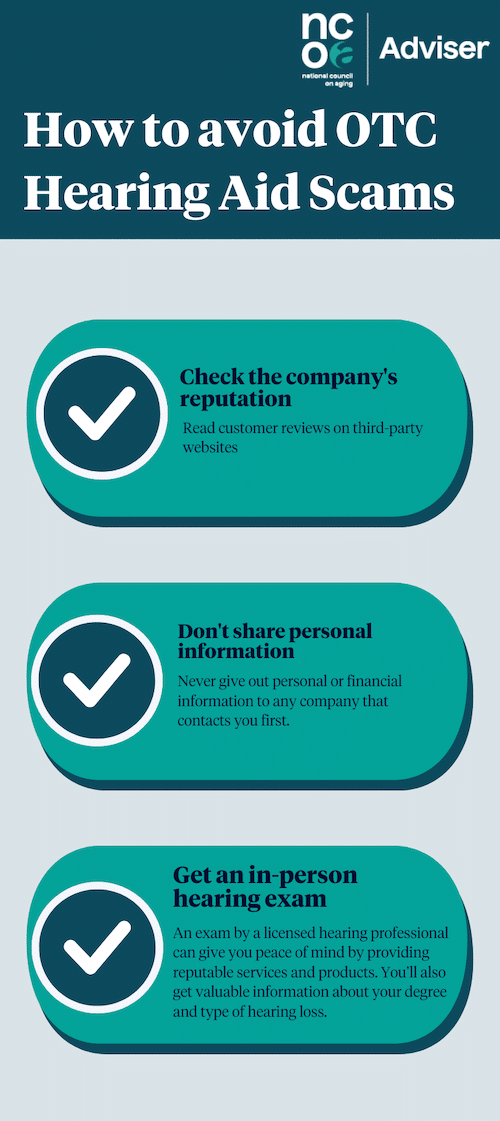
Check the company’s reputation
Read multiple reviews from trusted third-party sites, such as the Better Business Bureau and TrustPilot, to see if the company has a good reputation and to find out what other customers are saying about their products. If complaints have been filed against the company, how long did it take them to respond? Was the issue resolved to the customer’s satisfaction?
We have found that one common complaint among hearing aid customers is a refusal of hearing aid companies (such as Bossa) to refund their money when they return the hearing aids during the trial period. Steer clear of any company with a poor reputation for honoring trial periods, warranties, or requests for customer support.
Don’t share personal or financial information
It’s wise not to buy hearing aids from a company that calls or emails you first. You should always be the one contacting the company for information.
Consider an in-person hearing exam
By visiting a hearing care clinic for an in-person exam with a licensed audiologist or other hearing specialist, you’ll know you’re getting care from a professional who deals with reputable hearing aids. Keep in mind, though, a hearing exam does not obligate you to buy a hearing aid, even when the exam is free.
If the hearing specialist finds you have mild or moderate hearing loss and you want to try OTC hearing aids, you’ll be able to move forward with valuable knowledge about your hearing needs. On the other hand, the specialist may find you have more severe hearing loss, requiring prescription hearing aids. An in-person exam is the only way for a specialist to rule out underlying medical conditions or other causes for hearing loss that may need treatment other than hearing aids.
Some hearing clinics provide exams at no charge, although you’ll have to pay for a copy of your hearing exam results, also known as an audiogram. Many private insurance companies and Medicare Advantage plans include hearing benefits that will cover the cost of a hearing exam, and Medicare Part B also pays for hearing exams with a doctor’s order.7 Finally, U.S. veterans who receive VA health care benefits can receive hearing exams, hearing aids, and supplies at no cost.8
Whether you decide to have an in-person hearing exam or take an online hearing screening, checking your hearing level is just as important as checking your blood pressure. Hearing loss is linked to a multitude of negative health outcomes, and research has shown that the sooner it’s treated, the better the outcome.
For more information on FDA-approved hearing aids and a list of reputable brands, read our review of the best rated hearing aids.
What are OTC hearing aids?
Hearing aids have been available to purchase directly from manufacturers for several years, but an official OTC hearing aid category wasn’t created by the FDA until August 2022. The FDA press release states the primary goal of establishing standards for OTC devices was “to improve access to hearing aids, which may in turn lower costs for millions of Americans.”9
From what the market has shown over the past several months, it’s safe to say the FDA hit the mark on both improved access and lower prices. OTC hearing aids, designed for people 18 and older with perceived mild to moderate hearing loss, can be purchased online and in many retail stores without a hearing exam, prescription, or fitting appointment with an audiologist.10
By opening up hearing aid sales to retail stores and pharmacies such as Walmart, Best Buy, CVS, and Walgreens, the FDA has made hearing aids more available to people who don’t shop online.
The price tag on many OTC hearing aids is considerably lower than prescription devices, too. Our team’s research has found OTC hearing aids range in cost from $100–$3,000 per pair, whereas prescription hearing aids generally start at around $1,500 and can go up to more than $7,000 per pair depending on power level, technology, and features.
Our focus group of hearing aid users said cost was their highest barrier to buying hearing aids, and 30% of respondents to our team’s survey of hearing aid users said price was the biggest challenge with their hearing aids, so this is an important factor for many people.
FDA OTC hearing aid regulations
A common misconception among both hearing professionals and the public is OTC devices aren’t “real” hearing aids. OTC hearing aids actually include the same working components as prescription hearing aids, are defined by the FDA as medical devices,12 and must meet federal guidelines for safety and efficacy.13
The FDA’s final rule on OTC hearing aids defines OTC devices and outlines the specifications manufacturers must meet to be sold as FDA-registered or FDA-cleared OTC hearing aids.14 Aimed at safety and efficacy, the new OTC hearing aid regulations include the following:
- Definition: Intended for people 18 and older with perceived mild to moderate hearing loss
- Design
- Insertion depth: How deep the hearing aid can sit in the ear canal
- Volume control: Users must be able to change the volume
- Output limits: Maximum volume the hearing aid can emit
- Labeling: Clear, understandable language that explains who the product is appropriate for and when consumers should be evaluated by a health care professional
- Conditions for sale and distribution: Verification of customers’ age is not required
- Quality system requirements: Companies must follow good manufacturing practices for medical devices15
To be sold as self-fitting devices (meaning the user can make fine-tuning adjustments), manufacturers must obtain FDA clearance as a Class II medical device, which is a step beyond standard OTC classification. You may also see this listed as a 510(k) premarket notification in the FDA database.16
What is an FDA-registered hearing aid?
Because hearing aids are medical devices, the companies that produce and sell them must register annually with the FDA.

Some hearing aid websites advertise their products as FDA-registered. This term simply means the company is listed with the FDA. It does not mean the devices themselves are registered, or the FDA has issued an “approval, clearance, or authorization of that facility or its medical devices,” according to the FDA’s website.17
You can learn more about FDA registration and clearance of medical devices, as well as different classes of medical devices, on the FDA website.18
FDA-cleared self-fitting OTC hearing aids
In addition to FDA-approved registration as OTC hearing aids, some devices qualify for classification as FDA-cleared self-fitting OTC hearing aids.19 Self-fitting models have undergone further testing to demonstrate that users can program them at home, similar to how a hearing professional would in a clinic.
Are there FDA-approved hearing aids?
Not all OTC hearing aids require FDA 510(k) clearance. Luna explained that air-conduction hearing aids, which make up the majority of OTC and prescription hearing aids, are considered Class I or Class II medical devices. Class III devices, which include higher-risk hearing solutions like cochlear implants, always require FDA clearance.
Be wary of any companies that advertise “FDA-approved hearing aids,” as this isn’t a real marketing claim companies can make.20 “Most hearing aids for sale online will be FDA-registered. Only OTC hearing aids that have successfully completed an FDA 510(k) premarket clearance as self-fitting OTC hearing aids can make the claim to be ‘FDA-cleared,’” said Luna.
Let’s take a closer look at what sets OTC hearing aids apart from prescription hearing aids.
OTC hearing aids vs. prescription hearing aids
Traditional hearing aids, now called prescription hearing aids, are those sold at hearing care clinics in conjunction with an in-person hearing exam by an audiologist or hearing instrument specialist.21 They require a prescription for purchase and a fitting appointment for the hearing specialist to program them for your hearing loss profile.
One of the most notable differences between OTC and prescription hearing aids stands out in the FDA’s definition of OTC hearing aids as devices that are “intended for people 18 years of age and older who have perceived mild to moderate hearing impairment.”22
“Perceived” is different from “diagnosed.” Anyone who perceives they have mild to moderate hearing loss can buy OTC hearing aids without a diagnosis. This is why the FDA set forth the safety guidelines listed above: to ensure users won’t damage their hearing by turning a device up too loudly or inserting it too far into their ear.
Prescription hearing aids, on the other hand, do require a diagnosis of hearing loss based on an audiogram (results of an in-person hearing exam). Prescription hearing aids also require a fitting appointment by the hearing specialist because they operate using software the specialist will program based on your hearing exam results.
Along with these fundamental differences in definition and requirements for purchase, there are other gaps between the two types of hearing aids as well. See Table 1 and the following explanation for more details.
Table 1 OTC vs. prescription hearing aids
| OTC hearing aids | Prescription hearing aids | |
|---|---|---|
| Regulated by FDA | Yes | Yes |
| Degrees of hearing loss | Mild, Moderate | Mild, Moderate, Severe, Profound |
| Age of user | 18 and older | All ages |
| Prescription required | No | Yes |
| Ways to purchase | Online Retail stores Some hearing care clinics | Hearing care clinics |
| Average price per pair | $100–$3,000 | $1,500–$7,000 |
Both OTC and prescription hearing aids are regulated by the FDA and work by amplifying the sound frequencies users need to hear. But the differences stand out from that point on.
Degree of hearing loss
As noted above, OTC hearing aids can only treat mild to moderate hearing loss, while prescription devices can treat all degrees of hearing loss, from mild to profound.
Based on this, it may seem OTC devices are quite limited in their scope, but Frank Lin, MD, PhD, of the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, points out they can actually treat the vast majority of people with age-related hearing loss.
“The FDA put technical standards around [OTC hearing aids] that would allow for the maximum amount of utility for those devices for what is possible within a safe limit,” said Lin. “OTC hearing aids could be appropriate for about 90% of people with hearing loss.”
That’s good news for the 30 million Americans affected by hearing loss, many of whom aren’t able to afford prescription hearing aids.23
Age of user
Children under the age of 18 with hearing loss need to see an ENT doctor, audiologist, or hearing instrument specialist to have an evaluation for prescription hearing aids.
Ways to purchase
Prescription hearing aids are only available through hearing care clinics. While some are independently owned, others are part of larger networks. You can also buy prescription hearing aids at Sam’s Club and Costco hearing aid centers.
OTC hearing aids come with many more purchase options. This list gives you an idea of the places you can find OTC hearing aids:
- Online from the manufacturer
- Online from third-party retailers
- Online from the FSA/HSA store
- Stores where health care devices are sold, such as Best Buy, CVS, Hyvee, Walgreens, Walmart, and Victra Verizon
- Some hearing care clinics
Cost
OTC hearing aids tend to cost less than prescription devices. Based on our team’s research, we’ve found a general price range of $100–$3,000 per pair for OTC hearing aids, compared to $1,500–$7,000 for prescription hearing aids.
As you can see, there is quite a wide range, as well as some overlap, in prices. But prescription hearing aid prices start at 10 times the cost of the most budget-friendly OTC hearing aids. That could make all the difference for many Americans’ ability to afford treatment for hearing loss.
The wide range of prices for both types of devices is related to a number of factors, including:
- Battery type: Rechargeable hearing aids usually cost more than those that use disposable batteries.
- Features: Advanced functionality like Bluetooth streaming, hands-free calling, directional microphones, and self-fitting capabilities will add to the cost.
- Sound processing and listening settings: The more channels a hearing aid includes, the more sound frequencies it can process. Some hearing aids come with only a few listening settings, while others allow you to select from six or more and even fine-tune within each setting. Both of these make for a better listening experience, but they also increase the price.
- Hearing aid services included with purchase: Some hearing clinics include hearing aid cleanings, repairs, and other maintenance in the purchase price of their prescription hearing aids.
- Warranty coverage: Some, but not all, prescription hearing aids come with warranties that are longer and more comprehensive compared to OTC devices.
For more information on each of these factors and more pricing information on specific hearing aid models, see our review of the best hearing aids.
Hearing aids vs. PSAPs
You may have seen devices advertised as “hearables,” “hearing amplifiers,” or “earbuds for hearing enhancement.” Are these products hearing aids by another name? The short answer is no—they are considered personal sound amplification products (PSAPs).24
While hearing aids amplify only those sound frequencies the user needs help hearing (based on an app-based hearing screening or audiogram), PSAPs amplify all sounds. They are typically used by people with normal hearing to make sounds louder in certain environments, such as when bird watching or hiking.
PSAPs are not considered medical devices but rather consumer electronics, similar to headphones. As such, they aren’t regulated by the FDA or designed for people with hearing loss. The FDA published new guidelines for both hearing aids and PSAPs in August 2022 when they approved the OTC hearing aid category.25
As more manufacturers enter the hearing device market and label their products with an ever-increasing variety of names, it’s important to remember that hearing aids and PSAPs are two different devices with distinct uses. Take a look at Table 2 below for a quick overview.
Table 2 Hearing aids vs. PSAPs
| Hearing aids | PSAPs | |
|---|---|---|
| Designed to treat hearing loss | Yes | No |
| Regulated by FDA | Yes | No |
| Ways to purchase | Online, retail stores, hearing care clinics | Online or retail stores |
| Hearing exam or prescription required | No (OTC) Yes (prescription) | No |
The FDA’s website also provides more information on the difference between PSAPs and hearing aids.26
How to tell if hearing aids are FDA-compliant
The best way to tell if a hearing aid company has complied with FDA regulations is to use the search tool in the FDA’s medical device database. There, you can find a listing of both hearing aid manufacturers and hearing aid models registered with the FDA, as well as their product classifications.27
As noted above, labeling for OTC hearing aids must be easy to understand. In part, this means they should clearly state the term “hearing aids” on the package. Products that list only “hearing enhancement” or “improved sound quality” with no mention of hearing aids are likely PSAPs rather than true hearing aids.
Finally, if an OTC hearing aid is advertised as a self-fitting device, it should be listed as such in the FDA database.
What to consider before buying OTC HAs
Read this section to learn which factors you should think about before purchasing OTC hearing devices.
Are you a good candidate?
If you’re 18 or older and have mild to moderate hearing loss, OTC hearing aids may meet your needs. Children and people with severe or profound hearing loss will need an evaluation by an ENT doctor or hearing professional before buying hearing aids.28
In addition, if you have any of the following symptoms, see an ENT doctor for an in-person exam:
- Pain, injury, or drainage affecting one or both ears
- Dizziness or balance problems
- One-sided (unilateral) hearing loss
- Buzzing, ringing, or other constant sounds in one or both ears
- A clogged feeling in either ear

If you experience a sudden loss of hearing in one or both ears, go to the nearest emergency department for immediate medical attention.
Your budget
Hearing aid prices have dropped since OTC devices hit the market in late 2022. But at several hundred to several thousand dollars per pair, they’re still a prohibitively expensive purchase for many people.
Think about how much you can comfortably afford to spend on a pair of hearing aids. A quality pair should last about five years, and many OTC brands offer one- to three-year warranties that cover defects and repairs. Warranty lengths vary, though, so it’s important to find out the details before you buy.
For more information on ways to save money on hearing aids, see our article about affordable hearing aids.
Comfort level with DIY adjustments and remote care
One of the differences you’ll find between OTC and prescription hearing aids is that while prescription hearing aids come with in-person support from a hearing professional, most OTC hearing aids only offer remote support.
They’re also meant to be set up by the user at home, so you’ll need to be comfortable using the manual, website, and remote help from the company if needed to get your hearing aids going.
Is an app required?
Most hearing aids can be adjusted using either the buttons on the hearing aid or with a mobile phone or tablet app. But there are quite a few variations on this theme. Some hearing aids don’t come with an app at all, while others require an app to change the listening settings and for remote adjustments by the hearing aid company’s support staff.
Connectivity options
All prescription hearing aids come with full Bluetooth connectivity for app-based adjustments, audio streaming, and hands-free calls. OTC hearing aids vary in how much connectivity they offer, though.
Below are some of the levels of connectivity you’ll find among OTC brands:
- Bluetooth connection to an app for adjustments and remote care
- Bluetooth streaming ability for music and other audio
- Hands-free calling (check compatibility with your Apple or Android device before purchasing)
- Telecoil (for connecting to sound systems in public places)
Other features
Finally, think about other features you may want in a hearing aid, such as rechargeable batteries, water resistance, and advanced sound processing features, such as:
- Directional microphones (to help you focus on speech and other sounds in front of you)
- Feedback suppression (to reduce whistling noises)
- Digital noise reduction (to cut down on background noise)
Bottom line
OTC hearing aids can be an affordable solution for millions of Americans with hearing loss. But it’s important to be educated about potential red flags that may point to a scam and to know the signs of reputable companies that make quality hearing aids and provide reliable customer service.
Frequently Asked Questions
Unfortunately, scams exist in the hearing aid market just as they do in other industries.
The best way to protect yourself from an OTC hearing aid scam is to watch out for the red flags listed above. If you do suspect a company of being disreputable, it’s important to report any suspicious or fraudulent activity to the FDA in order to protect yourself and other hearing aid customers in the future.
If you are contacted by any company trying to sell hearing aids, asked for personal or financial information, or given high-pressure sales tactics, you may be the target of a hearing aid scam. It’s also important to buy only from reputable companies that have a positive reputation, clearly state “OTC hearing aids” on their labeling, offer a trial period with a money-back guarantee for you to try out the hearing aids, and give you several options for contacting customer support after your purchase.
The most reliable way to see if a hearing aid is registered with the FDA is to check the FDA database for medical device registration.
Have questions about this review? Email us at reviewsteam@ncoa.org.
Sources
- Federal Register. Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids. Aug. 17, 2022. Found on the internet at https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- U.S. Food and Drug Administration. Are There “FDA Registered” or “FDA Certified” Medical Devices? How Do I Know What Is FDA Approved? March 3, 2021. Found on the internet at https://www.fda.gov/medical-devices/consumers-medical-devices/are-there-fda-registered-or-fda-certified-medical-devices-how-do-i-know-what-fda-approved
- International Journal of Audiology. Why Do People Fitted with Hearing Aids Not Wear Them? March 2013. Found on the internet at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3665209/.
- Hearing Loss Association of America. Consumer Protection Laws. May 2013. Found on the internet at https://www.hearingloss.org/wp-content/uploads/ConsumerProtectionLaws.pdf
- University of California San Francisco. Hearing Loss Treatments. Found on the internet at https://www.ucsfhealth.org/conditions/hearing-loss
- U.S. Food and Drug Administration. Reporting Allegations of Regulatory Misconduct. Sept. 14, 2021. Found on the internet at https://www.fda.gov/medical-devices/medical-device-safety/reporting-allegations-regulatory-misconduct
- Medicare.gov. Hearing and Balance Exams. Found on the internet at https://www.medicare.gov/coverage/hearing-balance-exams
- U.S. Department of Veterans Affairs. Rehabilitation and Prosthetic Services. Found on the internet at https://www.prosthetics.va.gov/psas/hearing_aids.asp
- U.S. Food and Drug Administration. FDA Finalizes Historic Rule Enabling Access to Over-the-Counter Hearing Aids for Millions of Americans. Aug. 16, 2022. Found on the internet at https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- Johns Hopkins Medicine. Over-the-Counter Hearing Aids: Frequently Asked Questions. Found on the internet at https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/hearing-aids/over-the-counter-hearing-aids-faq
- Federal Register. Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids. Aug. 17, 2022. Found on the internet at https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- U.S. Food and Drug Administration. Over-the-Counter (OTC) Medical Devices: Considerations for Device Manufacturers. Aug. 25, 2021. Found on the internet at https://www.fda.gov/medical-devices/products-and-medical-procedures/over-counter-otc-medical-devices-considerations-device-manufacturers
- Federal Register. Code of Federal Regulations. Prescription Devices. March 10, 2023. Found on the internet at https://www.ecfr.gov/current/title-21/chapter-I/subchapter-H/part-801/subpart-D/section-801.109
- Federal Register. Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids. Aug. 17, 2022. Found on the internet at https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- Code of Federal Regulations. Quality System Regulations. March 10, 2023. Found on the internet at https://unblock.federalregister.gov/
- U.S. Food and Drug Administration. Product Classification. March 13, 2023. Found on the internet at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPCD/classification.cfm?ID=1714
- U.S. Food and Drug Administration. Are There “FDA Registered” or “FDA Certified” Medical Devices? How Do I Know What Is FDA Approved? March 3, 2021. Found on the internet at https://www.fda.gov/medical-devices/consumers-medical-devices/are-there-fda-registered-or-fda-certified-medical-devices-how-do-i-know-what-fda-approved
- U.S. Food and Drug Administration. Learn if a Medical Device Has Been Cleared by the FDA for Marketing. Dec. 29, 2017. Found on the internet at https://www.fda.gov/medical-devices/consumers-medical-devices/learn-if-medical-device-has-been-cleared-fda-marketing
- Code of Federal Regulations. Self-fitting air-conduction hearing aid. March 10, 2023. Found on the internet at https://www.ecfr.gov/current/title-21/chapter-I/subchapter-H/part-874/subpart-D/section-874.3325
- U.S. Food and Drug Administration. Overview of Device Classification. Sept. 4, 2020. Found on the internet at https://www.fda.gov/medical-devices/device-advice-comprehensive-regulatory-assistance/overview-device-regulation
- American Academy of Audiology. Audiologists vs. Hearing Instrument Specialists vs. ENTs. Found on the internet at https://www.audiology.org/consumers-and-patients/what-is-an-audiologist/audiologists-vs-hearing-instrument-specialists-vs-ents/
- U.S. Food and Drug Administration. FDA Finalizes Historic Rule Enabling Access to Over-the-Counter Hearing Aids for Millions of Americans. Aug. 16, 2022. Found on the internet at https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- National Institute for Deafness and Communication Disorders. Quick Statistics About Hearing Loss. March 25, 2021. Found on the internet at https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing
- U.S. Food and Drug Administration. Hearing Aids and Personal Sound Amplification Products: What to Know. Jan. 12, 2023. Found on the internet at https://www.fda.gov/consumers/consumer-updates/hearing-aids-and-personal-sound-amplification-products-what-know
- U.S. Food and Drug Administration. Regulatory Requirements for Hearing Aid Devices and Personal Sound Amplification Products. Aug. 16, 2022. Found on the internet at https://www.fda.gov/media/87330/download
- U.S. Food and Drug Administration. Hearing Aids. Jan. 12, 2023. Found on the internet at https://www.fda.gov/medical-devices/consumer-products/hearing-aids
- U.S. Food and Drug Administration. Establishment Registration and Device Listing. March 13, 2023. Found on the internet at https://www.accessdata.fda.gov/scrIpts/cdrh/cfdocs/cfRL/rl.cfm
- U.S. Food and Drug Administration. How to Get Hearing Aids. Nov. 18, 2022. Found on the internet at https://www.fda.gov/medical-devices/hearing-aids/how-get-hearing-aids
